For the ever-shrinking world of micro-electronics, I hear the size of the wires themselves are a tricky problem to handle. For one thing, how do you place and solder a wire that's only a dozen atoms across? I don't work in micro-electronics (or any kind of electronics) so that's all I'll say about that.
However, a new nanowire has recently been connected between two tiny electrodes. It's so tiny it can only be seen under an atomic force microscope, and this is what it (well, a whole series of them) looks like:
They conduct nearly as well as copper, and made extremely low-resistance contact with the electrode surfaces they're connecting. Even better? They're self-assembling with an easy to control "on" switch, and charging the electrodes before triggering the self-assembly means the wires build themselves between the charged electrodes and nowhere else.
That AFM picture is actually of many nanowires bridging the gap between two electrodes, as shown in the diagram beside it. The surface of the electrodes themselves is quite rough-looking, compared to the surface of each nanowire that reaches across the gap.
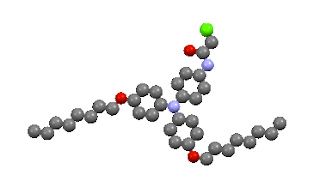
The nanowires are made of a class of compounds called triarylamines. One of the molecules they tested looks like this:
These molecules float around in solution until light knocks an electron off of that central nitrogen (purple), at which point the remaining electron sticks to the central nitrogen in another molecule.
The three carbon (grey) rings arranged around a central nitrogen (purple) stack like plates on other molecules just like this one, and they keep building. The dangly bits around the outside stick out of the plate stack—or maybe wrap themselves around the plate stack somehow; that AFM photo made the wires look very smooth.
But not just any molecule in this group works. The dangly bits make a difference: some of the compounds they tested did not form nanowires, even though they had the same three-ring core. Here's one of the molecules that didn't stack itself into wires:
From the looks of it, if the compound can stack itself into wires, it does so when light catalyzes the reaction by knocking an electron off some of the central nitrogens. Electrons being what they are, these free radicals try to complete the pair by grabbing at other nitrogens, but no matter how many they grab and form into that plate stack, there are still missing electrons. And electrical conductivity happens when electrons have a place to go, which they do wherever there are missing electrons.



No comments:
Post a Comment